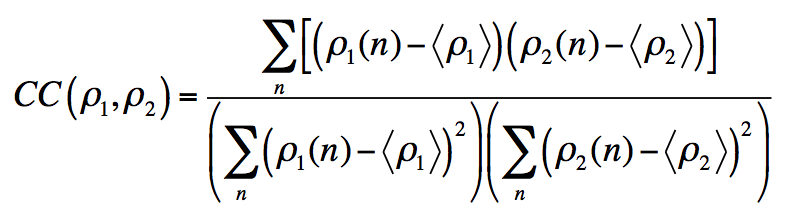| Ramachandran favored, allowed, outliers |
A two-dimensional graph of the phi and psi backbone angles. Due to steric
constraints, only certain combinations are possible (Ramachandran et al. (1963) J Mol Biol 7:95-99) and the he plot is therefore divided into "favored", "allowed", and "outlier" regions. Validation reports indicate the percentage of phi/psi angle pairs in these areas. A well-refined structure should have 98% of residues favored, and less than 0.2% outliers, although it may be difficult to obtain these statistics at lower resolutions. Note that the expected distribution varies depending on residue type and environment. The results page shows the Ramachandran plot for all
non-Pro/Gly residues. |
| Rama-Z score |
The Ramachandran Z-score characterizes the shape of the backbone angle distribution in the Ramachandran plot (Hooft et al., 1997). Even if a refined model has satisfying Ramachandran statistics in terms of fractions of residues belonging to favored/allowed/outlier regions, the distribution of backbone dihedrals can be improbable (Sobolev et al., 2020). A normal backbone protein backbone geometry results in Rama-Z values between -2 and 2. A less likely yet possible distribution has absolute Rama-Z values between 2 and 3. A Rama-Z score with an absolute value above 3 corresponds to an improbable Ramachandran distribution. |
| Clashscore |
Validation statistic used in Molprobity and Phenix validation tools. Two atoms
have a severe clash, if they overlap by more than 0.4 Å. The clashscore represents the number of severe atomic clashes per 1000 atoms. A well-refined structure should have a clashscore below 20; oftentimes clashes will require manual rebuilding to fix. Clashes can be visualized in KiNG or Coot as "Probe dots"; these are generated by Molprobity or the Comprehensive Validation in Phenix. |
| rmsd values |
Protein structures are refined using prior knowledge about the molecule.
Geometrical parameters of amino acids, such as bond lengths, bond angles,
or dihedral angles are known from high resolution X-ray structures of
polypeptides. These parameters are expected to be similar
in proteins and are therefore restrained to stereochemical standards. The
root mean square deviation (rmsd) of the refined geometry from these dictionary
values gives in indication if the model conforms to the standards. |
| Bond rmsd |
Root mean square deviation of covalent bond lengths from dictionary values. |
| Angle rmsd |
Root mean square deviation of bond angles from dictionary values. |
| Chiral rmsd |
Root mean square deviation of chiral volumes. |
| Planar rmsd |
Root mean square deviation of atoms from a plane, such as for the peptide
plane or aromatic side chains. |
| Dihedral rmsd |
Root mean square deviation of torsion (dihedral) angles from dictionary
values. |
| Cβ deviations |
Number of instances where the distance between the observed location
of the Cβ atom from its ideal position exceeds 0.25
Å. This distance represents distortions around the
Cα atom, f.ex. if the backbone and/or sidechain are
misfit, the Cβ atom may be moved far from the ideal
position to compensate. A deviation of more than 0.25 Å is
considered to be a validation outlier and should be inspected during the
validation process. |
| Rotamer outliers |
In the context of model validation (Phenix, Molprobity) rotamers refer to the set
of dihedral angles in amino acid side chains. Some combinations are preferred
while certain combinations are not possible, due to steric and other atomic
interactions. Phenix uses MolProbity’s Ultimate Rotamer-Library (Hintze, B.J. et al., Proteins: Struc Func Bioinf 84, 1177-1189 (2016)) to categorize
rotamers as "favored", "allowed" or "outlier" (similar to Ramachandran angles). The vast majority of sidechains in a finished structure should be favored unless the density very clearly supports an outlier conformation. |
| Minimum non-bonded distance |
The shortest distance between two atoms which are not covalently bound
or form other bonded interactions. |